Water hardness measurement (°dh) and monitoring in water softening plants
Through water treatment reduce energy costs?
The hardness of water is formed by dissolved salts. In natural waters, the hardness is formed mainly from magnesium and calcium hydrogen carbonate and sulfate, which were dissolved from the soil and / or aquifers. The hardness of the water is higher, the more of these salts are dissolved in the water..
Because of the regionally very different nature of the geological subsurface, are thus most diverse water hardnesses and compositions to be found. In this context, one divides the water hardness in the following areas:
| < 4 Degree of German hardness (°dH) | Very soft water |
| 4 – 7 Degree of German hardness (°dH) | Soft water |
| 7 – 14 Degree of German hardness (°dH) | Medium hard water |
| 14 – 21 Degree of German hardness (°dH) | Hard water |
| > 21 Degree of German hardness (°dH) | Very hard water |
Terms in water hardness monitoring
Magnesium and calcium ions, for example, can enter water through simple dissolution processes, such as the dissolution of gypsum.
However, the majority of water hardness is formed as carbonate hardness by dissolution of lime (CaCO3) or dolomite (Ca-Mg mixed carbonate). Under the action of carbonic acid (CO2), the formation of soluble hydrogen carbonates (HCO3-) occurs. The CO2 originates predominantly from the soil and is produced in particular by the microbial decomposition of organic substances.
Other input pathways include dissolution by acidic components of precipitation or by nitric acid from nitrification resulting from agricultural fertilization.
In the technical context, a wide variety of terms are used to represent the specific properties of water as a measurable quantity.
Total hardness, carbonate hardness, temporary hardness, transient hardness, residual alkalinity, m-value or acid capacity up to a pH of 4.3 (KS 4.3)
Acid capacity (KS) up to pH 8.2 (p-value, KS 8.2)
Base capacity (KB) up to pH 4.3 (-m-value, KB 4.3).
The total hardness is defined as the sum of all alkaline earth ions; these are mainly calcium and magnesium.
In solution, these are “paired” with chlorides, sulfates, carbonates and other anions.
Determination methods of water hardness measurement
The best known and most accurate determination method for total hardness is the determination based on complexometric titration.
Through this, a highly accurate ACTUAL value analysis is possible..
Depending on the area of application and treatment process, the monitoring of water hardness is of crucial relevance for a wide variety of processes.
In measuring devices for limit monitoring, such as the Testomat 808, a fixed amount of indicator, which is designed by the chemical formulation for a specific “absorption capacity” of Ca2+ and Mg2+, is used. If an excess is added to the water sample by the dosed amount, a green coloration of the sample water is produced.Conversely, if the concentration of Ca2+ and Mg2+ in the water sample to be analyzed is higher, a red coloration of the water is produced.
By means of the instruments of our Testomat family we offer you instruments for limit value monitoring as well as for the exact determination of the ACTUAL value.
The most commonly used process for water softening is the ion exchange process.
Mode of operation of a softening plant
In the ion exchange process, the water to be softened flows through a container filled with cation exchange resin, which is also colloquially known as a column. Inside the container, the calcium and magnesium ions contained in the water are replaced by the sodium ions bound in the cation exchange resin.
The water loses the hardness-forming calcium and magnesium ions and thus becomes soft.
However, this process only works until the cation exchange resin has released all its Na+ ions.
Once the cation exchange resin is “exhausted,” no more calcium and magnesium ions can be absorbed and the water hardness rises again. To prevent stagnant contamination, 3% Bio Resin is added to the resin.
To provide continuous soft water for processes, softening plants are often operated with at least two columns. In this process, the first column is used until the cation exchange resin is exhausted, while the second column is on standby.
When the absorption capacity of the first column for calcium and magnesium ions is reached, the plant automatically switches over to the second column.
Technically, this switchover can be made, for example, on the basis of mathematical calculations.
Here, a continuously constant raw water quality (proportion: calcium and magnesium ions in the raw water) is assumed in order to determine the theoretical useful time of the column.
The disadvantage of this volume-based control is that fluctuations can only be reacted to by means of safety factors and thus regeneration does not take place at the optimum time or on the basis of the actual degree of depletion.
By means of the devices of our Testomat family, we offer devices for limit value monitoring as well as for the exact determination of the ACTUAL value. The precise determination of the water hardness downstream of the softening plant ensures an effective plant control, which ensures an optimal use of the absorption capacity of the individual columns and thereby simultaneously contributes to savings effects on the operating cost side.
In addition to monitoring softening plants, other indicators are available for process monitoring of total hardness up to 25° dH.
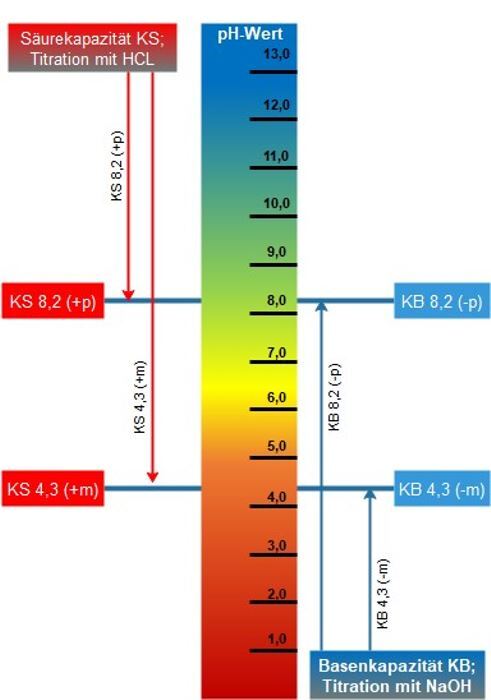
The acid capacity is a measure of the buffer capacity (pH stability) of water against acids. In practice, the two following acid capacities are used here for the implementation of process engineering processes.
In terms of water hardness, the concentration of the anion hydrogen carbonate (HCO3-) is of particular importance. This concentration is called carbonate hardness, temporary hardness, transient hardness, residual alkalinity, m-value or acid capacity up to a pH of 4.3 (KS 4.3). The designation m-value is based here on the use of the indicator methyl orange, which has its turnover point at a pH of 4.3.
The determination of the carbonate hardness (m-value, KS 4.3) is done by determining the hydrochloric acid binding capacity. For this purpose, a defined amount of water sample is titrated with hydrochloric acid (c = 0.1 mol/l) to pH 4.3. By using the indicator methyl orange, a highly accurate determination is possible. The acid consumption in ml corresponds here to the hydrogen carbonate concentration in mval/l. Multiplication by the fixed conversion factor 2.8 gives the result in degrees of German hardness (°dH).
The “acid capacity (KS) up to pH 8.2” (p-value, KS 8.2) covers all alkaline components of water that form hydroxide ions, such as free bases, as well as the first hydrolysis stages of the alkali and alkaline earth salts of weak, polyvalent acids (e.g. carbonates, phosphates, silicates). The term p-value is based here on the use of the indicator phenolphthalein. It indicates how much acid a water sample absorbs up to the transition point of the indicator phenolphthalein (pH 8.2).
The determination is carried out by determining the hydrochloric acid binding capacity up to pH 8.2 For this purpose, a defined quantity of water sample is titrated with hydrochloric acid (c = 0.1 mol/l) up to pH 8.2. By using the indicator phenolphthalein, a highly accurate determination is possible. The acid consumption in ml corresponds here to the hydrogen carbonate concentration in mval/l. Multiplication by the fixed conversion factor 2.8 gives the result in degrees of German hardness (°dH).
The base capacity (KB) up to pH 4.3 (negative m-value, KB 4.3) indicates the sum of the content of strongly acidic anions (Cl, SO4, NO3) and serves, for example, their determination after the water sample has been passed through a strongly acidic cation exchanger. In contrast to the softening system, which replaces calcium and magnesium ions with sodium ions, here the exchange is for H+ ions. Through this exchange, the water is “acidified”. The determination is carried out by titration with NaOH (c = 0.1 mol/l) until the pH value 4.3 is reached. The indicator methyl orange, whose transition point is at a pH value of 4.3, is also used in this method.
Water Hardness Online Measuring Instruments
Water hardness online measuring device Testomat EVO TH and LAB.
State-of-the-art instrument technology with new product advantages.
For hardness measurement in water treatment plants, the new EVO TH is the future-proof further development of the traditional Testomat 2000 and replaces it. The LAB is the version reduced to the essentials.
Request individual offer directly online
Request an offer directly via the watch list.
After consultation with you, we will be happy to create your individual offer with these products or products that fit your needs.
Our experts will contact you prepared.