Measurement of chlorine and chlorine dioxide
The hygienic requirements to be met by water are therefore specified in various laws and regulations:
Drinking water
“Water for human consumption must be free of pathogens, fit for human consumption and pure. This requirement is considered to be fulfilled if the generally recognized rules of technology are observed during water extraction, water treatment and distribution, and the water for human consumption complies with the requirements of §§5 to 7.” (Drinking Water Ordinance 2001 (TrinkwV 2001, 2nd section §4).
Permissible disinfectants for drinking water according to the Drinking Water Ordinance 2001, 3rd section, §11 “Treatment substances and disinfection processes” are:
- Chlorine (gas) (Cl2)
- Chlorine dioxide (ClO2)
- Ozone (O3)
Bath water
“Swimming or bathing pool water in public baths or commercial establishments must be of such a nature that its use is not likely to cause damage to human health by pathogens. Swimming or bathing pools, including their water treatment plants, are in this respect subject to monitoring by the health authority” (§11 Bundes-Seuchengesetz, BSeuchG).
Permissible disinfectants for bathing water according to DIN 19643-1 (Treatment of swimming and bathing pool water) are:
- Chlorine (gas) (Cl2)
- Sodium hypochlorite (NaOCl)
- Calcium hypochlorite (Ca (OCl)2)
In addition to the applications already mentioned, chlorine-based media are also used in cooling towers. These provide an ideal environment for the growth of microorganisms. Left unchecked, this biofouling interferes with the proper functioning of the cooling tower and can lead to the formation of Legionella colonies, which are discharged if the system is not operated properly. Application of microbiocides such as chlorine inhibits the growth of these organisms. Microbiological control of cooling tower water is usually accomplished by the addition of oxidizing microbiocides, particularly chlorine dioxide. For effective dosing, precise monitoring of residuals is required. Chlorine residuals must also be monitored in the effluent to ensure compliance with the respective local regulations.
The Testomat LAB Chlorine uses the DPD method for determining the concentration of free chlorine in accordance with DIN EN ISO. 7393-2. One advantage of this colorimetric measuring method over the sensory measuring methods is that it is not subject to changes in temperature, pH, chlorine concentration and works stably even under pressure and flow rate fluctuations.
The Testomat LAB Chlor was specially developed for monitoring total chlorine or free chlorine in the field of cooling water conditioning, whereby the parameter to be monitored is determined by selecting the reagent set used. With the extended measuring range of 0 – 5 mg/l, processes of shock chlorination as well as the associated distortion rate (reduction of the concentration of free chlorine) can also be reliably monitored. For optimized process integration, the so-called measuring phase mode is available in addition to continuous analysis at a fixed measuring interval. In this operating mode, an analysis sequence is triggered for a period of 10 minutes to 12 hours.
In combination with water, chlorine forms hypochlorous acid (HOCL), which is also colloquially referred to as free chlorine. Only this is responsible for the disinfection performance. It should be noted here that the formation of HOCL is very strongly dependent on the pH value. The following conversion rates can be assumed as a rough guide:
- pH value 6.5: approx. 85 % HOCl.
- pH value 7.5: approx. 50 % HOCl.
- from pH value 8: hardly any formation of HOCl.
For the above reason, the pH value is always monitored in addition to the chlorine value, which serves as a further control parameter for the addition of “chlorine” and thus an increase in the oxidation potential (or redox potential).
If a chlorine-releasing substance is added to a water that contains neither organic matter nor ammonium compounds, this water contains only free chlorine. However, if the above-mentioned compounds are present in the water, less free chlorine is measured, but additional bound chlorine is measured instead. In simple terms, the following reactions take place:
- Free chlorine oxidizes organic substances.
- A preliminary stage of complete oxidation is the bonding of chlorine to amine (this is contained in every protein substance).
- The chloramine thus formed is called bound chlorine.
Chloramines (combined chlorine) are responsible for the chlorine odor (indoor pool odor). Above a certain concentration, they cause skin and eye irritation. The content of combined chlorine should therefore be kept as low as possible.
The disinfection effect of combined chlorine is much lower than that of free chlorine.
The partial removal of the bound chlorine is achieved by shock chlorination, which should be performed regularly.
Chlorine dioxide (ClO2) dissolves as a gas in water. Hydrolysis as with chlorine does not occur. The disinfection effect of chlorine dioxide is based on an oxidation reaction of the undesirable water constituents, in which chlorine dioxide is reduced to chlorite (ClO2 -). Chlorine dioxide cannot be stored in compressed form or in higher concentrated solutions because of its explosiveness. For this reason, ClO2 is prepared at the point of use from sodium chlorite (NaClO2) and chlorine (Cl2) or sodium hypochlorite (NaClO) or from sodium chlorite and hydrochloric acid (HCl).
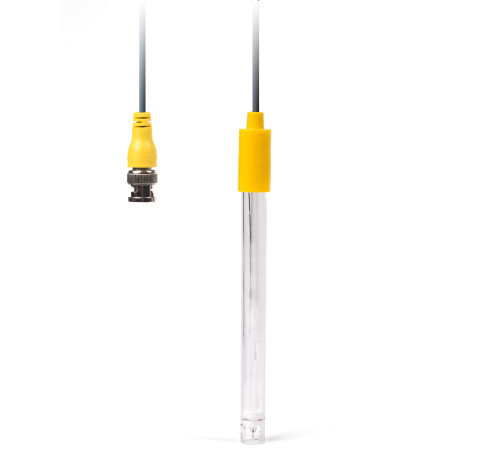
Monitoring of the required chlorine concentration within water treatment processes is carried out, for example, using so-called redox electrodes.
The measurement of the redox voltage is a potentiometric method using a redox electrode. Almost no current may flow during the measurement so that the chemical composition of the measured solution remains unchanged. The sensor is the redox electrode. It consists of a measuring electrode and a reference electrode. The redox electrode assumes the redox potential during the measurement. For oxidizing solutions, the potential of the redox electrode is more positive and for reducing solutions more negative than that of the reference electrode.
The determination of redox potential is significant for water testing in connection with the control of chlorination of swimming pool water.
The absorbance is proportional to the dye concentration present or the free chlorine concentration present. In the absence of iodide ions, only the free chlorine is detected.
If potassium iodide is subsequently added to the test sample, the chlorine substitution products present in the sample (combined chlorine) release a stoichiometric amount of iodine. The result is the total chlorine concentration.
The difference between total chlorine and free chlorine gives the combined chlorine. In addition to the DPD method, there are two other methods for chlorine determination: a titrimetric method with DPD as indicator (DIN EN ISO 7393-1) and an iodometric method (DIN EN ISO 7393-3).
For the determination of chlorine dioxide, the method according to DIN 38408-G5 (a titration) is used. This method is based on the oxidation of DPD by ClO2. The dye formed is decolorized by titration with ammonium iron (II) sulfate solution. The concentration of chlorine dioxide is calculated from the consumption of the solution. Alternatively, photometric determination of ClO2 using the DPD method is also possible. As with chlorine, the concentration of ClO2 is calculated from the absorbance with a chlorine dioxide-specific factor (1.9).
The previously presented determination methods are not continuous measurement methods, but samples are taken at specific times and individual measurements are carried out. The DIN 19643 specifies continuous measurement, control and recording of free chlorine and pH for the treatment of swimming and bathing pool water. For the control of the disinfectant concentration, it is advantageous if an electrical signal is continuously available that is proportional to the disinfectant concentration. This signal can then be used as an input signal for controlling a dosing system for the disinfectant, i.e. the concentration can be controlled fully automatically.