Why conductivity measurement cannot replace water hardness measurement
Conductivity measurement as a substitute for water hardness measurement?
What does water hardness actually mean?
- The term water hardness refers to the proportion of calcium and magnesium in the water
- Dissolved natural substances: calcium & magnesium
- There is a division into different degrees of hardness:
The more lime there is in the water, the “harder” it is.
Water that is too hard usually requires the use of a water softening system.
Water absorbs many dissolved natural substances on its way through the ground, including calcium and magnesium.
The hardness of water depends on the content of calcium and magnesium ions. Hard water contains more minerals compared to soft water.
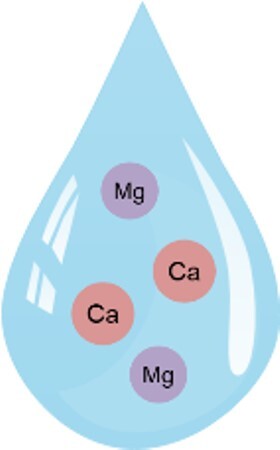
**Hard water contains more magnesium and calcium than soft water. These elements are important minerals for the body.
It is therefore not a problem for health.
**Hard water is not a problem for washing machines, as all modern detergents contain sufficient softeners in the correct doses. Kettles or coffee machines can be easily descaled with diluted citric acid.
| < 4 Degree of German hardness (°dH) | Very soft water |
| 4 – 7 Degree of German hardness (°dH) | Soft water |
| 7 – 14 Degree of German hardness (°dH) | medium hard water |
| 14 – 21 Degree of German hardness (°dH) | Hard water |
| > 21 Degree of German hardness (°dH) | Very hard water |
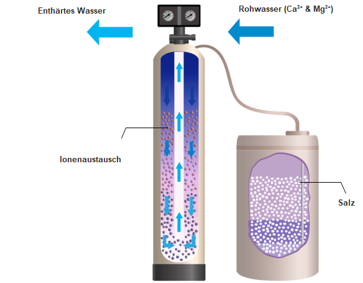
The water softener removes lime from drinking water
Areas of application:
- Industrial applications
- Boiler feed water
- Cooling towers
- Food industry
- Pharmaceutical industry
- Hospitals
- Households
- Hotels …
The ion exchange process, which removes the lime, calcium and magnesium from the water and replaces them with easily soluble neutral salts, e.g. sodium.
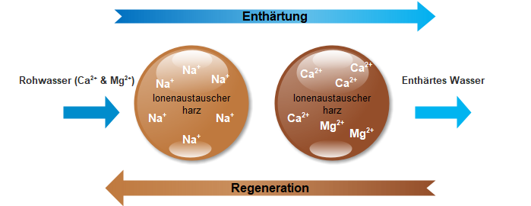
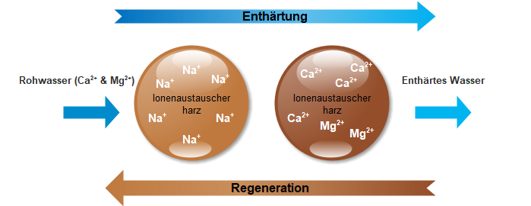
When the ion exchange resin is exhausted, the salt, through the regeneration process, is transferred from a storage tank to a tank containing the resin and the lime is completely “flushed out”.
**This lime-containing solution runs into the waste water pipe. This ensures continuous decalcification.
**The limit value of the sodium content in the water here is 200 mg/l according to the law (Drinking Water Ordinance).
Does the salt concentration in the water increase after water decalcification?
The assumption that there is more salt in the water due to a water softener is wrong.
Only the statement that you increase the sodium content is correct.
Sodium and salt are not the same. If no ions are exchanged, the salt content is not increased.
Through ion exchange, the chemical composition of the water remains unchanged.
The total salt content is hardly changed by the exchange.
It is often assumed that the salt solution is in the drinking water after decalcification. This is not the case.
- the sodium content increases, that is correct.
- however, sodium and salt are not the same.
- ion exchange replaces Ca and Mg with Na, so the total salt content of the water remains unchanged in this process.

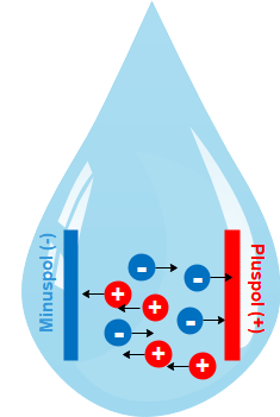
What does electrical conductivity mean?
Conductivity is the ability of a substance (liquids, solids, gases) to conduct electric current and heat.
- A quantity of physics
- The unit of electrical conductivity
- The unit of electrical conductivity is Siemens per meter [S/m] in SI units.
- In water, one usually speaks of microsiemens per centimeter [µS/cm].
The net movement of charged ions produces an electric current in water and ionic materials as well as in fluids and is called ionic conductivity.
The conductivity depends on the concentration and type of ions. The temperature of the medium also has a great influence.
In water or ionic fluids, a net movement of charged ions can occur.
This phenomenon produces an electric current and is called ionic conductivity.
**Because the electric current is transported by dissolved ions, the conductivity increases with increasing ion concentration.
The electrical conductivity of the water
This table gives information about the LF of ultrapure water, 0.05 mS/cm, tap water 500 mS/cm, seawater 50 mS/cm.
- The conductivity of water is a property that indicates the general quality of the water.
- The conductivity measurement does not permit any specific interpretations.
- Only different dissolved substances in water, such as H+, Na+, Ca2+, Mg2+, OH-, Cl-, SO42-, … are producers of conductivity.
- Conductivity only gives an indication of the total amount of ions dissolved in water and not accurate chemical analysis of the dissolved substances.
- The more ions dissolved in the water, the higher the conductivity of the “water”.
- Basically, pure water contains very few ions, i.e., it does not conduct electricity.
- The lower the conductivity, the fewer ions are dissolved in the water.
But the measurement of conductivity does not give any information about the chemical analysis of the dissolved substances, only an indication of the absolute number of dissolved particles in the water.
**There are also substances dissolved in water, such as hormones, fungicides or pesticides, which do not conduct electricity and are therefore not detected by the conductance.
**The comparison between seawater and distilled water is also interesting. Here, the electrical conductivity comes about because of the dissolved ions in the water. Seawater has a very high proportion of salt, which dissolves in the water. These ions transmit the current. In distilled water, there are no dissolved ions, so virtually no current can flow. Therefore, the electrical conductivity of seawater is much higher than that of distilled water.

Typical conductivity at 25°C
| Ultrapure water | 0,05 μS/cm |
| Tap water | 500 μS/cm |
| Seawater | 50 mS/cm |
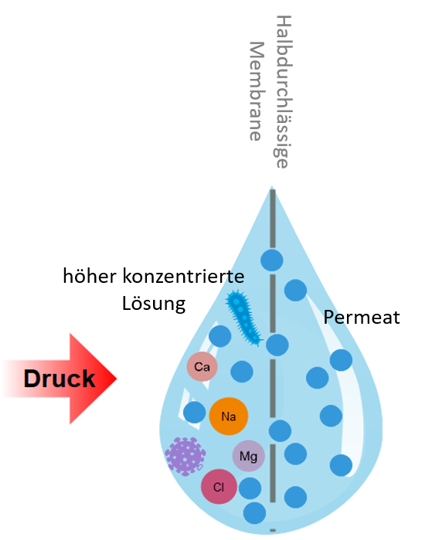
What is the water desalination?
A physical process to remove dissolved salts from water.
The standard process of desalination is the Reverse osmosis process.
Semi-permeable membranes are used in reverse osmosis.
These membranes are permeable to water molecules, but not to the dissolved salts, organic matter, TOC (total organic carbon), bacteria and viruses they contain.
With an increase in pressure, the water is forced through these membranes and desalinated.
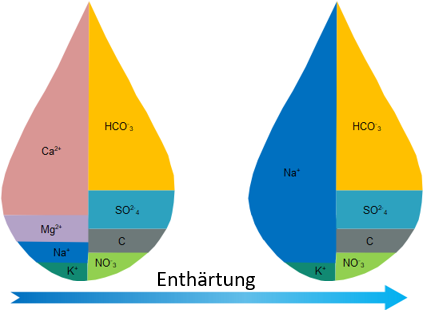
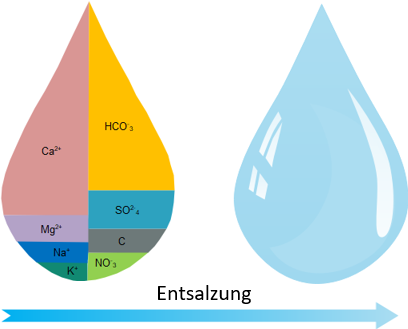
The change in conductivity due to softening and desalination?
Dhe difference between softening and desalination lies in the conductivity of the water already mentioned.
During softening, calcium and magnesium ions are exchanged for Na ions in the water, so that the salt content and thus the electrical conductivity do not change.
Unlike softening, desalination plants actually remove all salts from the water (reverse osmosis process).
Unlike softening, desalination plants actually remove all salts from the fill water.
Conductivity measurement cannot replace water hardness measurement
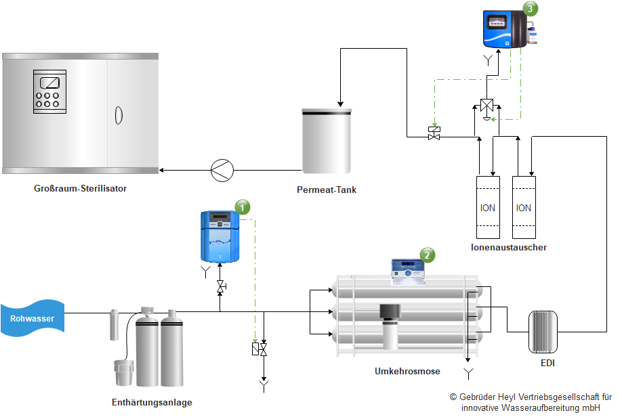
- Testomat EVO-TH: Residual hardness monitoring
- SOFTMASTER ROE: Conductivity monitoring for reverse osmosis systems
- Testomat 808 SiO2: monitoring of SiO2 concentration
Typical conductivity at 25°C
| Conductivity (µs/cm | |
| Raw water sample | 513 |
| Behind the softener | 504 |
| Behind the osmosis plant | 10,8 |
In this table you can see:
The conductivity hardly changes due to the softening plant.
But downstream of the reverse osmosis plant it is reduced.
That’s why we need a Testomat downstream of the softening plant, for example, to monitor the residual hardness. And after the reverse osmosis the conductivity monitoring.