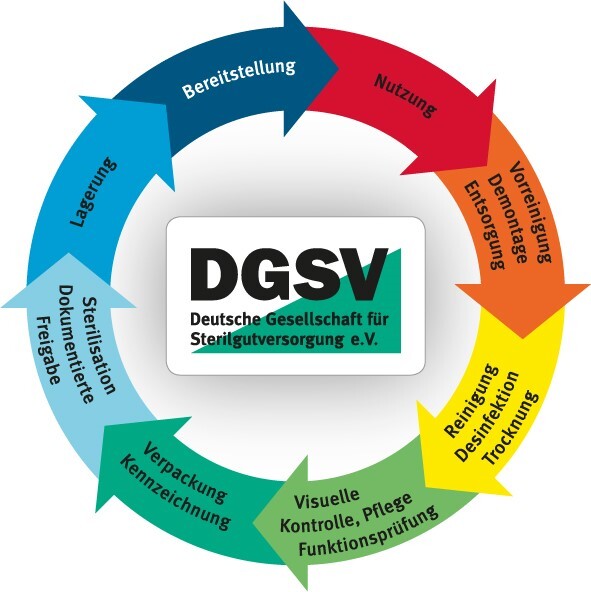
Sterile processing for optimum safety in the clinic AEMP
Optimization of the sterile goods reprocessing process in clinics
Increasing process reliability and reducing undesirable follow-up costs by taking a holistic view of the sterile goods reprocessing process in hospitals.
Cleaning, disinfecting and sterilizing – sterile reprocessing of medical instruments is an important task to prevent the transmission of germs or pathogens.
The Medical Devices Implementation Act (MPDG) and the Medical Devices Operator Ordinance (MPBetreibV) legally regulate the use of hygienically perfect and professional medical instruments on patients.
The cause of the transmission of germs and pathogens may be due, among other things, to the sterile goods reprocessing process.
In Germany, there are approximately 30,000 to 35,000 nosocomial infections with MultiResistant Pathogens (MRP) per year. 1,500 cases or 0.3% of all nosocomial infections in Germany are due to multidrug-resistant pathogens, which are resistant to almost all classes of antibiotics.
According to the RKI, there are a total of about 400,000 to 600,000 nosocomial infections and about 10,000 to 20,000 deaths caused by them. The number of deaths in Europe attributable to nosocomial infections is estimated at around 91,000 cases per year.
The cause of nosocomial infections and MRE may be rooted, among other things, in the sterile processing area, where instrument quality plays a significant role in transmission.
- Stress cracks and wear
- Corrosion, chrome plating and surface residues
- Functional testing in the AEMP and in the OR area
- Steam qualities of the steam sterilizers
- Cleaning performance of washer-disinfectors (RDG) / container washers (CWA)
- Process water qualities
Many years of practical experience have shown that the limit values for feed water and condensate specified in the currently valid standard continue to result in premature wear of the system technology, sterile materials and instrumentation and therefore do not meet the hygienic requirements for a load-free, sterile medical device and the protection of patients and investments.
Water treatment therefore represents an essential element in the instrument cycle. Not all water is the same, and not all steam is the same.
The sterilization process and all associated elements of media generation are of elementary importance for the overall process. In addition to generating clean steam, the water produced is also used for rinsing and cleaning processes.
The interaction of a wide variety of technical components poses a challenge and requires very precise coordination and consideration of the entire process, including the provision, use and reprocessing of the instrumentation.
Insufficient information on the overall process, the condition of the entire system including the pipe network, plant technology, treatment capacities lead to problems and thus damage to the treated goods. As a consequence, this results in a risk to patient safety.
Steam sterilization represents a very sensitive and demanding part of medical device reprocessing. Process water and steam within the sterilizers (autoclaves) directly transfer negative influences to the instrumentation and represent a latently high and cost-driving process risk in terms of hygiene, which is actually High risks for patient safety results in.
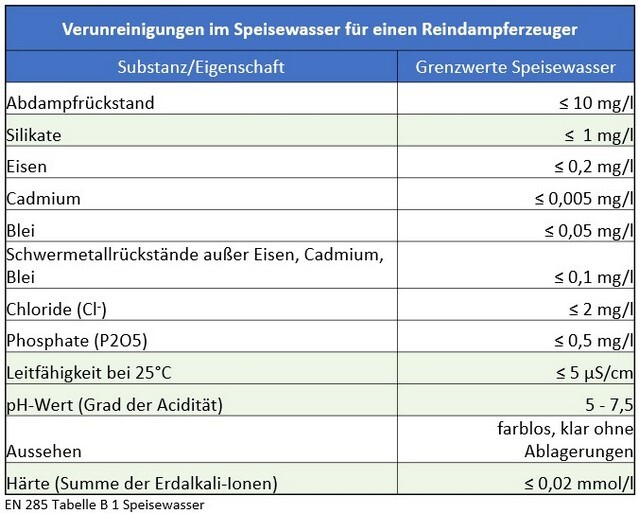
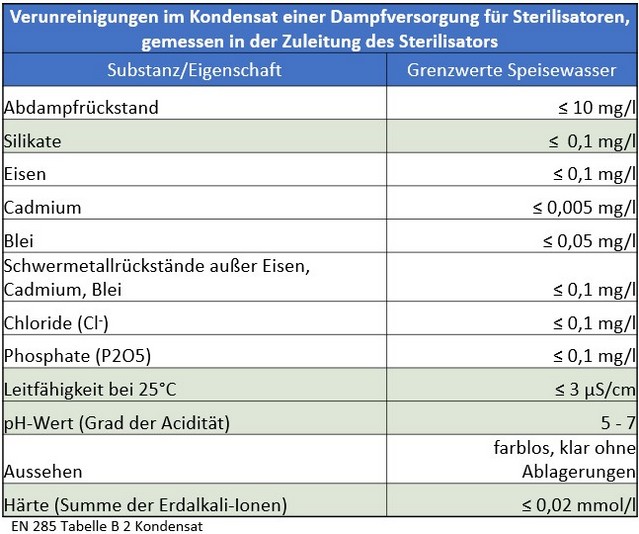
In day-to-day practice, it is apparent that the standards set out in the current DIN EN 285 The limits set for feed water and condensate in this process can lead to premature quality defects in the equipment technology, the components and the overall system for sterile processing.
However, there is currently no legally binding standard for the planning of water treatment for the sterilization of instruments. Planning and design are carried out on the basis of or using DIN EN 285.
The Medical Devices Act (MPG) and the Medical Devices Operator Ordinance (MPBetreibV) regulate the use of hygienically unsound and improperly prepared instruments on patients in accordance with §26 MPG.
In addition to negative consequences for patient safety, contaminated or defective medical devices lead to high costs in the area of new acquisition and/or repair.
For hospitals with at least 4 cutting disciplines and different numbers of beds, the table below shows examples of possible new acquisition costs for surgical instrument sets as well as average repair proportions of the instrument set:
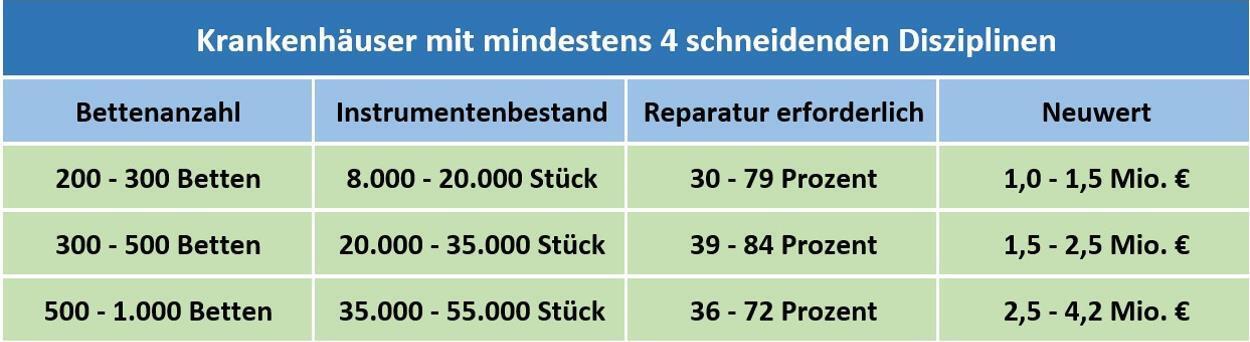
Overview of instrument requirements and cost commitment
Basis: Around 200 stock analyses in the years 2002 – 2021

Therefore, the following question must first be answered to maintain patient safety and ensure the economic efficiency of the sterile processing by the relevant groups of people:
- Are the costs in the area of repairs and repair replacement services of medical devices in the hospital still in good proportion?
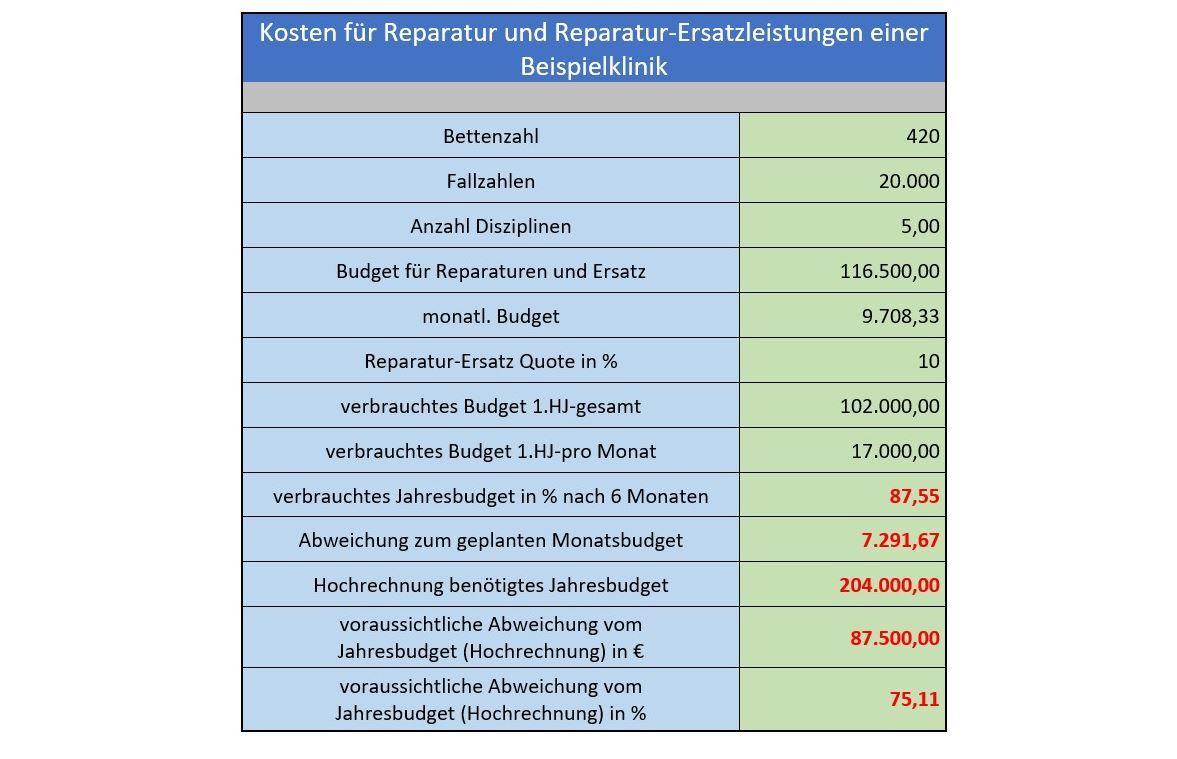
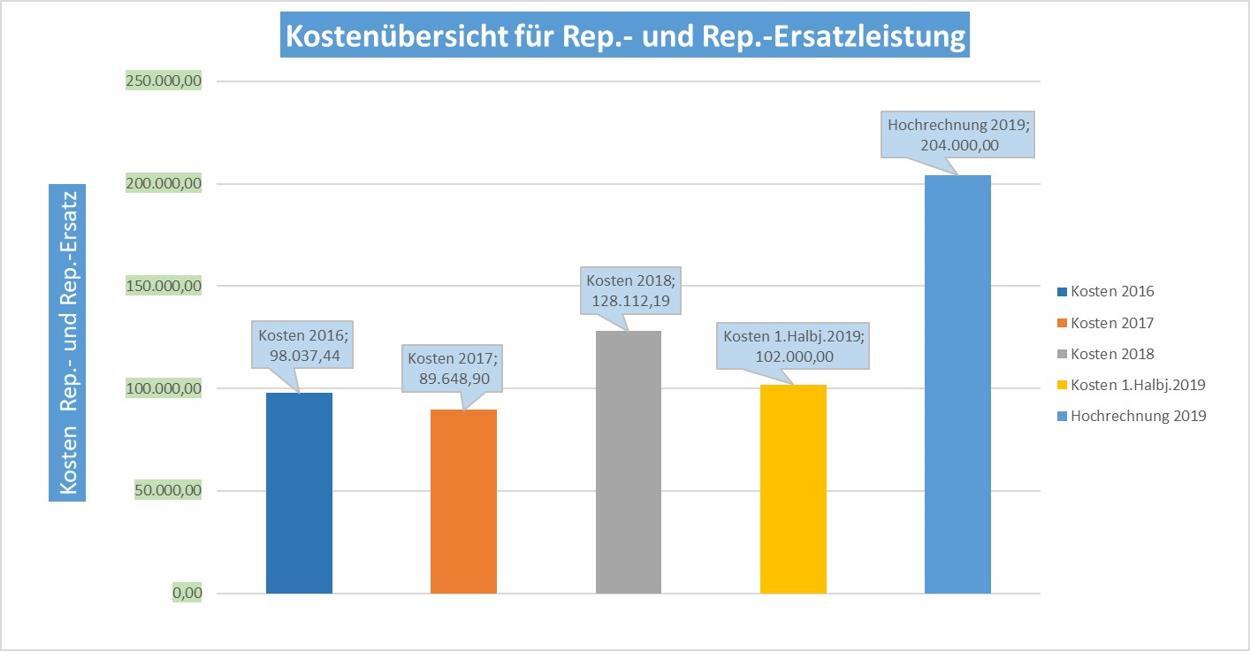
As a result of the inventory of instruments, it should be noted:
- The allocated budget is almost exhausted after only 6 months.
- A significant overrun of the annual budget is expected.
The question must be asked, what are the triggers for this?
A massive deviation from the budgeted costs indicates problems in the handling of the instruments, the preparation and the media associated with them, as well as their generation. The above results clearly indicate an acute need for action.
Damage to the instrumentation shortens the service life from approx. 15 years to < 8 years with poor media quality. Poor media quality is directly related to the management costs of the instruments.
The entire sterile goods reprocessing process of the clinic should be fully assessed and analyzed immediately. This can prevent a possible total loss of the existing instruments and equipment technology, so that patient safety is not negatively affected in the long term.
The example described above clearly shows that continuous monitoring of the sterile preparation process is essential to prevent damage to instrumentation and equipment technology, thereby taking patient safety into account and avoiding incalculable costs.
- Who monitors the sterile goods reprocessing process and can detect process errors at an early stage?
- Who assesses whether the process is still in good as-is condition?
Responsibilities and processes in an AEMP
The above considerations regarding the repair and repair replacement costs for instrument sets have already shown the high relevance of media quality. Ensuring this and all related processes is the responsibility of the departments named below:
- Management and staff of the AEMP (formerly ZSVA)
- Hygiene officers and hygiene specialists
- Equipment and house technicians
- Operating and functional areas
- Logistics management
as well as
- chemical suppliers, instrument manufacturers, instrumentation manufacturers, and water purifiers.
The management plays an essential role in this. It is responsible for the successful economic management of the healthcare facility, in compliance with the quality criteria to ensure instrument and patient safety. Taking into account the specific personal liability risk as an operator of healthcare facilities in accordance with the Medical Devices Implementation Act (MPDG), the managing director and all of the persons named above are responsible for ensuring the proper reprocessing of medical devices.
Negative effects due to improperly prepared instrumentation, such as postponing or canceling surgery, which means a negative economic impact, are avoided.
The complex interplay between water purifiers, suppliers of equipment technology, end users and other players involved in the sterile processing process becomes clear on the basis of the numerous stakeholders. Complex overlaps in responsibilities as well as the existing process interfaces require interdisciplinary cooperation between the groups of people involved.
Goals / process requirements
Ensuring the required media safety in the course of process water treatment and thus optimally minimizing the process risk for the reprocessing of medical devices as well as limiting the risks for patient safety to a minimum represent the primary objective. Cost-effectiveness in the sterile goods reprocessing process by identifying potential savings, taking into account individual obstacles and structural hurdles, is taken into account in the process design.
The focus here is on avoiding and preventing costs for the renovation or new acquisition of equipment and plant technology, right through to the renewal of complete supply systems.
How should the sterile goods reprocessing process be assessed to ensure that the above objectives are achieved?
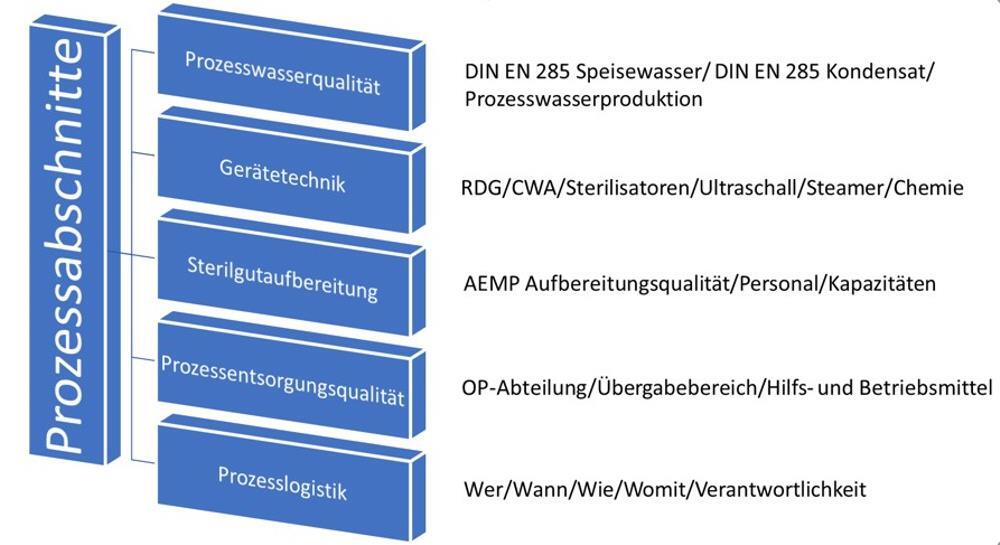
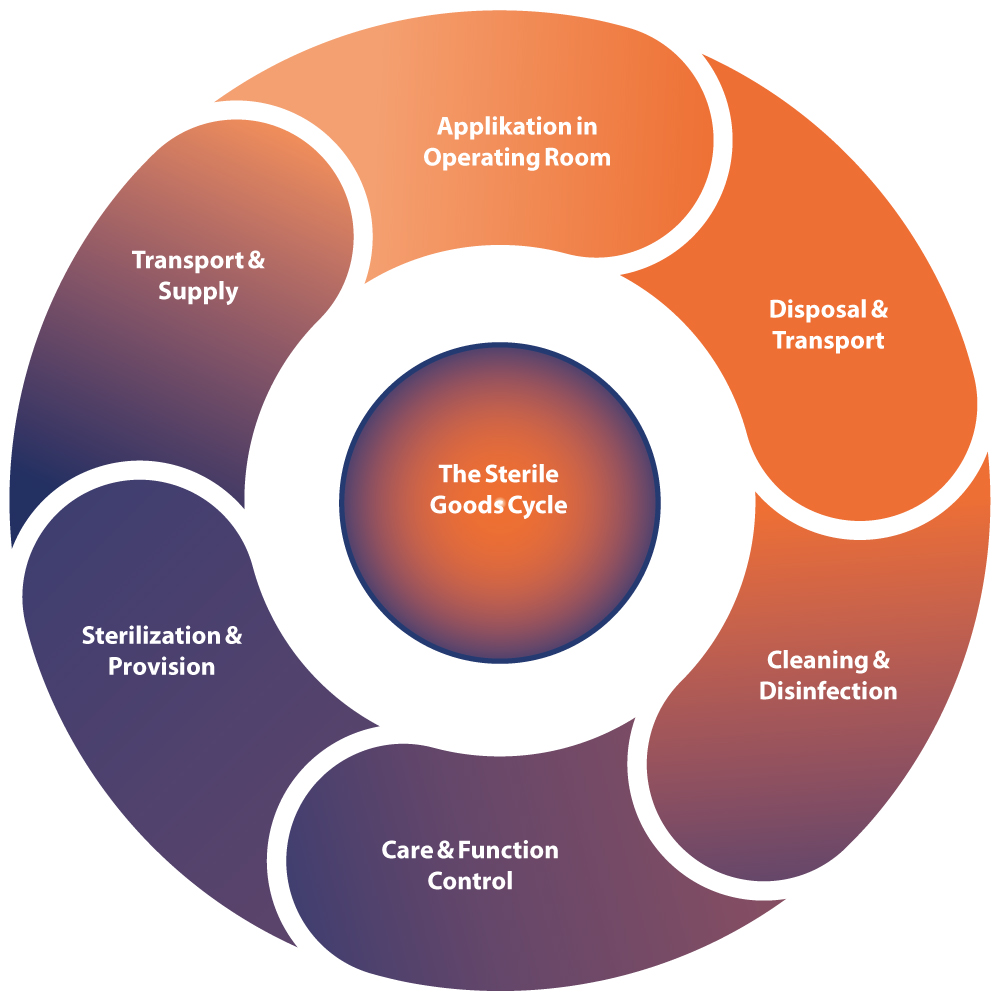
This diagram illustrates the individual process steps in sterile processing which contribute significantly to achieving good instrument quality.
Starting with the process water, the following points must be taken into account when planning a new water treatment system or evaluating an existing one:
- What type(s) of use or purpose(s) does the treated water serve?
- Are there any standards and/or further specifications regarding the quality for the application?
- What is the water quality at the feed point?
A water treatment can never be transferred 1 to 1 from location A to location B!
In order to evaluate the overall process for the medium water, comprehensive analysis is necessary, starting with water treatment and extending to the actual sterilization process.
The Instrument Reprocessing Working Group (AKI) has been working for many years on the topic of a holistic view of the instrument cycle..

The associated brochure already contains comments as well as recommendations that are significantly below the limit values of DIN EN 285.
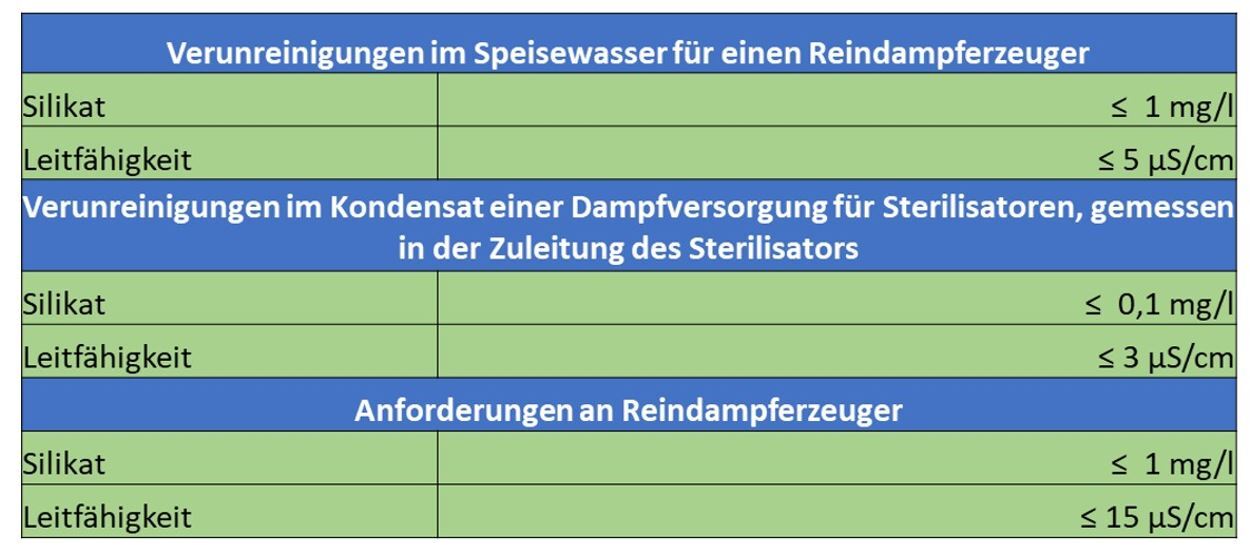
„The so-called silicate slip already occurs at conductivities > 1µS/cm!
In order to reliably achieve a value of 0.1 mg/l in the condensate, the feed water to the sterilizer should not contain more than 0.4 mg/l SiO2!
In order to obtain reproducibly stain-free instruments, the silicate content should be permanently below 0.4 mg/l.
The Quality monitoring of the demineralized water via electrical conductivity control is for an identification insufficient, as the silica does not impart conductivity to the water.“
Extract 11th edition 2017; pages 21-22
The limit values of drinking water differ massively from the limit values permitted by process engineering. At the same time, depending on the water source, the chemical composition or the proportion of dissolved substances in the water is always different. In order to comply with the necessary limit values, the water treatment must therefore be adapted to the source material “drinking water”.
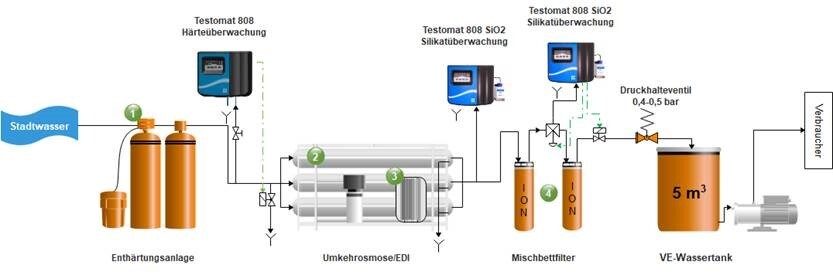
For the treatment of drinking water to feed water for a steam generator, the following process stages are primarily used:
- Softening plant
- Reverse osmosis
- Electrodeionization
- Blending bed
Für die Bedarfserhebung (-berechnung) werden folgende Daten benötigt:
- Anzahl Reinigungs- & Desinfektionsgeräte (RDG)
- Anzahl Dampfsterilisatoren
- Anzahl Containerwaschanlagen (CWA)
- Betriebsmodell des Standortes (Schichtbetrieb 12, 16 …24 Stunden)
Alle benannten Anlagen haben spezifische Abnahmeleistungen pro Stunde und spezifische Laufzeiten in Minuten (abhängig vom Sterilisationsprogramm & Gerätetyp)!
If additional consumers are supplied via the same water treatment system, the water demand must also be surveyed.
From the data collected, the first relevant process variables can be approximated:
- max. quantity taken per minute (relevant for the design of the pressure boost, among other things)
- Daily requirement of deionized water (relevant for design or evaluation of existing storage tanks).
An initial assessment of the existing plant concept against this background is already possible here.
Based on these quantities, a recalculation is now made for the individual treatment stages of the water treatment. The following questions must be taken into account:
- For how long must at least the emergency supply from the permeate tank be ensured?
- What is my maximum purchase quantity per minute?
For this consideration, the approach with a simultaneity factor of 90% has proven successful (90% of all existing customers request at the same time). - Which switching points of the level switches must be taken into account for water make-up?
The aim here is to find a balance between offtake and replenishment that ensures that the plants run for a sufficiently long time, but at the same time do not have too long downtimes. - What yields do the individual modules deliver?
A wastewater stream is generated in each treatment stage. The “loss quantities” must be taken into account in the plant performance. - What is the composition of my raw water before the first treatment stage?
The water analysis contains the decisive information for the selection of suitable treatment stages and related components. For example, the water hardness has a direct influence on the size of the softening plant to be selected. In turn, the output capacity of the softening plant is decisive for the selection of the reverse osmosis and thus also the EDI. Other relevant ingredients may include disinfection media and dissolved heavy metals.
Is the existing water treatment technology suitable to map the current demand for process water and, if applicable, to ensure already known future demand?
The bottom line is that all the individual components involved in the process
- Process water quality
- Piping systems
- RDG/CWA devices
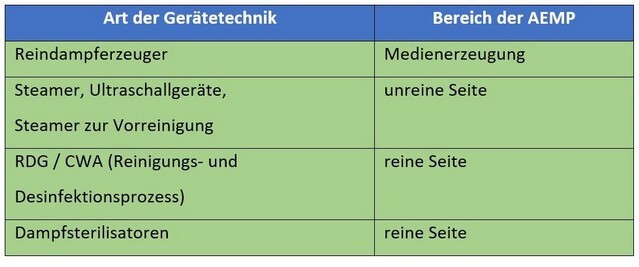
- Auxiliaries such as steamers and ultrasonic units
- Cleaning chemistry
- Steam sterilizers
- Pure steam generators and
- Feeding technology
decisively influenced by the quality of the feed water and the steam quality. If things are not right here, no stable and safe reprocessing process will be able to take place. In the sterile goods reprocessing process, in addition to the quality of the process water, the equipment technology and the design of the AEMP play an important role. reprocessing unit for medical products) plays a decisive role.
What equipment technology is required in the AEMP for sterile processing?
After the instruments have been used in the operating room, they are transferred to the reprocessing department.
In the course of reprocessing, the following essential activities are performed by the professionally trained personnel:
- Recording of the delivered medical devices in the ERP system
- Pre-cleaning of the instruments from adhering contaminants and deposits; if necessary, disassembly for the reprocessing process
- Loading of the transport equipment for the automated cleaning and disinfection process in the washer-disinfector and/or CWA
- Functional control and packaging, including packaging in sterile goods barriers (containers, flow, etc.) as well as any necessary replenishment from the sterile goods warehouse
- Sterilization
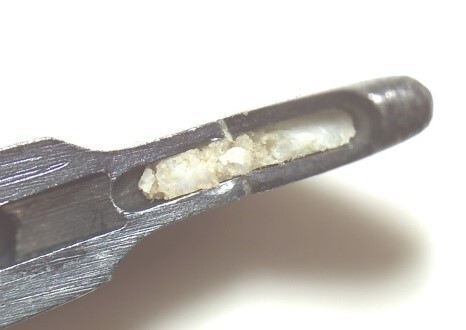
Example of insufficient cleaning performance of the WD (cartilage residues)
The arrangement and design of the AEMP with the above-mentioned equipment technology contributes significantly to the reprocessing result of the instrument set.
There are no clear, comprehensive regulations in the known technical literature. The DGSV publishes comprehensive recommendations for action, which are compiled and updated with the participation of experts in the individual fields. Among other things, the following publications have already been made for the construction or conversion of an AEMP:
Hygiene requirements for the reprocessing of medical devices
- Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM), 10/2012, in particular Annex 5 “Overview of requirements for reprocessing units for medical devices”.
- Recommendation for the reprocessing of medical devices, framework for uniform administrative action. Prepared by: Project Group “RKI-BfArM Recommendation” of the Working Group on Medical Devices (AGMP).
- DIN 1946:4, 2008 “Ventilation systems in healthcare buildings” – TRBA 250 – 2014
as well as
- longtime experiences
The focus of the recommendations is on structural requirements.
Practical experience has shown that deficiencies in sterile processing are often caused by insufficient spatial separation within an AEMP and insufficient involvement of users in the planning processes. Deviations from the above recommendations may result from the specific conditions in healthcare facilities themselves or from the special requirements within the AEMP.
The hygiene regulations and, if applicable, the monitoring regulations of the federal states must be observed, e.g. with regard to the involvement of the hygiene commission in structural or organizational changes with relevance to hygiene.
Basically are three areas and additional rooms are required.
- CLEANING AND DISINFECTION AREA (impure)
- Receiving zone with PC workstation if necessary
- Work section manual pre-cleaning
- Cleaning/disinfection
- if necessary, separation of the cleaning/disinfection sections Thermostable/Thermolabile
- Loading zone RDG
- if necessary, trolley washing area/trolley washing system
- Storage area for transport/loading trolleys
- if necessary, parking area for loading trolley for washer-disinfector
- for vertical connection
- Access to elevator “unclean
- adjoining rooms, if necessary dosing room (dosing center), if necessary cleaning room
- PACKING AREA (pure)
- Withdrawal zone RDG with release
- PC workstation
- Intermediate storage
- Packing and control stations, if necessary with PC workstation
- if necessary, separate workstations for low-germ goods/ goods to be sterilized, different packaging materials/ sterilization methods
- if necessary, storage area for loading trolleys and/or transport racks
- Loading zone for sterilizers
- Ancillary rooms, such as cleaning room and, if necessary, storage room for spare instruments, consumables/supplementary materials
- STERIL GOODS AREA (pure)
- Removal zone Sterilizers
- Cooling zone with release, if necessary with PC workstation
- Order picking
- if necessary storage zone and dispensing area
- with vertical connection
- Access to elevator “clean
In addition OTHER ROOMS such as social and office areas as well as CONNECTING AREAS between unclean and clean areas.
In addition to the structural requirements, the proper functioning of the equipment used is absolutely essential to ensure proper sterile processing.
Due to the introduction of water in the form of steam and ultrapure water for rinsing processes, the surfaces are in permanent contact with this medium. It is possible to ensure the necessary media quality by setting up a water treatment system that is adapted to the process. In this context, considerations must also be made with regard to the media distribution system. Special attention must be paid to the piping systems.
A frequent cause for the re-introduction of critical components into the properly treated water are impurities in the pipelines which, for example, result from:
- improper assembly (warm cutting to length instead of cold cutting to length, lack of deburring, unsuitable materials, etc.),
- insufficient maintenance of the equipment technology, and
- improper dosing of cleaning products.
This results in follow-up costs for the repair/maintenance of the equipment technology and media lines. Particularly cost-intensive are the pipeline rehabilitation and the reprocessing costs of the equipment technology, for example:
- the chamber cleaning of a sterilizer: approx. 10.000€.
- the cost of a new purchase of equipment technology
- container washing machine: 90.000€
- cleaning and disinfection device: approx. 60.000€
- clean steam generator: approx. 25.000€
- Sterilizer (12 STE): 70.000€
- Charging trolley: 2.500€ – 9.000€
to be accounted for.
If these costs are transferred to an exemplary model clinic, the cost model below results for reprocessing and new acquisition of contaminated equipment and feeding technology.
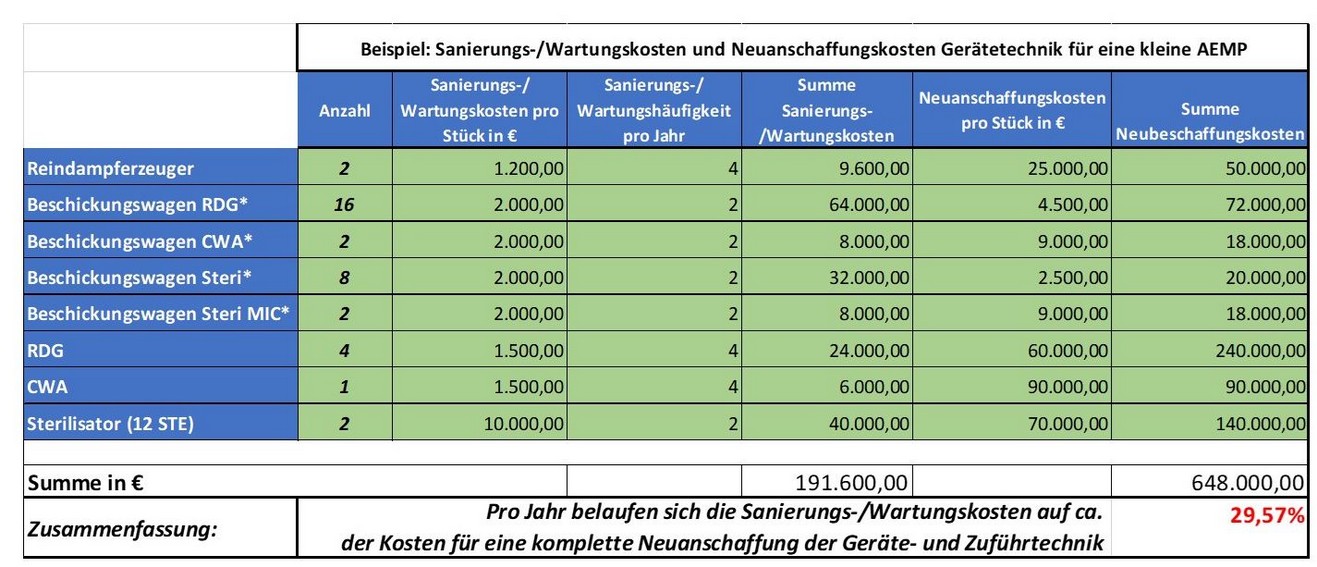
All avoidable risks serve to ensure the highest possible availability of the instrumentation and thus to ensure site and patient safety.
The reprocessing of used instruments is already significantly influenced by handling within the OR. In addition to damage during use, other factors have a direct impact on downstream treatment:
- Deposition in the instrument sieve
- Exposure to or contact with media from the surgical environment (e.g., saline solutions, Ringer’s solutions, etc.).
- Treatment with enzyme foam
- Service life of the instruments until sterilization
The AEMP specialists inspect the incoming sterile items on the unclean side and evaluate them for possible anomalies. If necessary, the instruments are dismantled and comprehensively pre-cleaned for the downstream cleaning, disinfection and sterilization processes.
In summary, it can be stated that part of a holistic approach is the installation of preventive measures through consistent risk management in the sterile goods reprocessing process as well as the identification of potential savings.
Particular attention is paid here to the value retention concept for medical devices. Decisive consideration is given, among other things, to the avoidance of new acquisition costs, repair costs and repair replacement costs. In this context, measures to reduce the procurement volume are defined (screen optimization) and the right balance between repair and new acquisition is reviewed. Important indicators in the consideration are the utilization times of the individual instrument groups in connection with the reconditioning & repair costs as well as replacement costs.
Reliable conclusions about the condition of instrument reprocessing can be drawn from these three elements without having to visit an AEMP. The comparison of repair and repair replacement costs based on the previous year’s values enables an assessment of whether the costs are within the expected range or whether there has been a disproportionate increase in costs.
The focus here is on the development of basic data in order to make decisions on a transparent basis, as well as the definition of quality criteria to achieve instrument and patient safety and fulfillment of the legal framework for action.
The collection, interpretation and evaluation of the individual data groups requires extensive knowledge of the entire process, including process engineering evaluation of plant, dosing and other preparation technology. In addition, close communication with procurement and controlling is required. In practice, it is precisely this complexity and variety of different topics and responsibilities that pose a challenge.
Our experts have extensive practical experience in the monitoring and moderation of process analysis in the field of sterile goods processing. Based on this experience, we have developed a portfolio of services tailored to the needs of our customers, which ensures effective support in this complex area. In particular, this includes the modules shown graphically below, which are applied according to the needs and wishes of the customer. To complete our range of services, we cooperate with accredited laboratories for the performance of accompanying laboratory tests and thus ensure the independence of our consulting services.
Our services
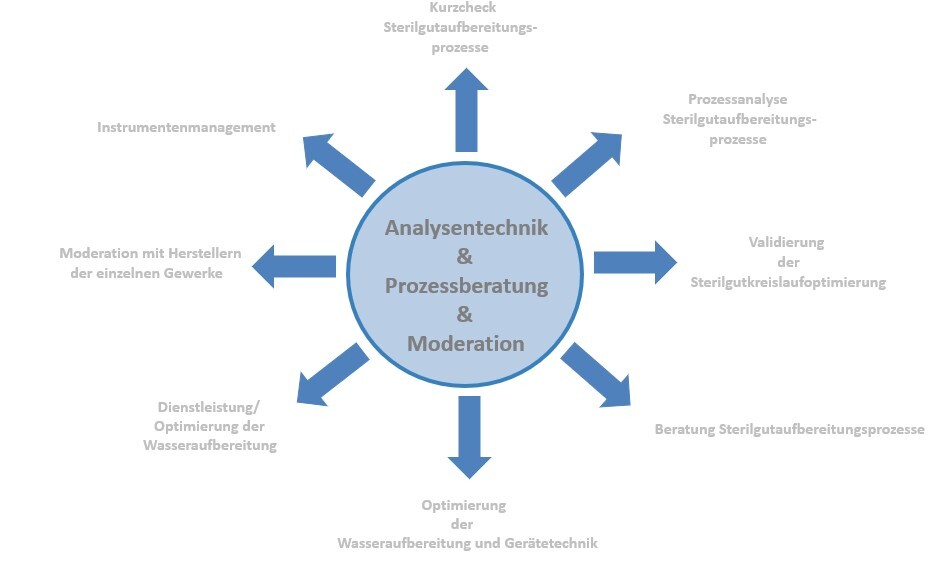
To ensure qualified and targeted project support, we accompany our customers step by step from the brief check, through process analysis, to training measures and workshops for employee qualification.
- Quick check of the sterile goods reprocessing processes
-
Process analysis of the sterile goods reprocessing processes
-
Validation of the sterile goods cycle optimizations carried out
-
Consulting and monitoring of the sterile goods cycle optimization processes
-
Training measures and workshops of the sterile goods reprocessing processes
The key principles of our service portfolio can be summarized as follows:
We analyze, evaluate and document the actual state of the water and steam treatment system
We analyze the sterile goods reprocessing processes and the quality of the instrumentation
We create individual recommendations for action
We accompany the implementation of the recommendations for action by our experts in the areas of water treatment, sterile goods processing and instrument management
From this, measures for process optimization can be derived, the extent of which depends on the size of the facility and the desired safety in the process. Services serve to permanently ensure the quality of instruments and processes and thus the highest good, patient safety.
In addition, targeted qualification measures should be installed to ensure the required quality and efficiency.
A possible optimization potential of a clinic depends primarily on the currently existing qualitative situations in the above-mentioned areas.
The sum of all individual measures, when consistently implemented and monitored, enables a significant increase in process reliability. Here, it is particularly important that the individual measures are implemented correctly and that validation is carried out to ensure the results achieved.
As a result, quality criteria are defined for assessing the existing hygiene measures. Based on this, recommendations for action for integration into the existing occupational health and safety measures, operating regulations and operating procedures of the sterile processing and hygiene process are shown.
The following questions should be answered to determine the individual implementation steps and their prioritization within the project matrix:
- What is the planning basis for future sterile processing?
- To what extent do I want to invest in the measures listed below?
- What degree of target achievement (safety) do I want to achieve in the end?
In order to achieve the required level of safety in the sterile goods reprocessing process, various measures must be implemented in water treatment, instrument logistics, the OR area, equipment technology, and general reprocessing.
Safety and efficiency in the entire hygiene and sterile goods reprocessing process are thus achieved through process analytics and the resulting integration of new technologies and working methods.
Summary
The costs of hygienically unsound medical devices are measured not only in terms of actual new acquisition costs, repair and replacement costs, but also in terms of the time required to sterilize the instruments in order to meet the specifications of the applicable guidelines. Furthermore, non-indirect monetary costs, such as the loss of patient safety, play a central role in the evaluation of the sterile processing procedure. Therefore, it is critical that the performance of the entire sterile processing system is maintained at all times.
Professional monitoring of water treatment and the associated early detection of performance degradation can prevent damage and reduce downtime and lost revenue.
Analysis and evaluation of the existing sterile goods reprocessing process including the creation of an individual concept for sustainable instrument reprocessing as well as the derivation of recommendations for action for all groups of people involved in the process.
This includes for example:
- Optimization of process water treatment
- Optimization of the media-carrying piping systems
- Optimization of equipment technology/feeding technology
- Optimizing chemistry
- Optimizing sterile goods logistics
- Optimization of screen content structures
- Optimization of the replenishment reserve
- Training measures for the AEMP/OP area
Based on the individual concept proposals, measuring and monitoring devices are installed at the water treatment plant and measures are defined in the sterile goods logistics and operating theater departments, depending on the desired degree of target achievement in terms of efficiency, safety and cost-effectiveness.
All avoidable risks serve to ensure the highest possible availability of the instrumentation and thus to ensure site and patient safety.