Redox potential / ORP value
The phenomenon of combustion was the first process in which the redox potential was demonstrated. It is assumed that every combustible substance contains a “fire substance” (phlogiston) and that this is released during combustion.
It was not until the middle of the 18th century that the French chemist Lavoisier recognized that oxygen was consumed during combustion. He gave the name oxidation to all processes in which a substance reacted with oxygen. As reduction he referred to the return of the metal oxide formed to its original state.
Many other reactions in chemistry are also outwardly no different from combustion, but they do not involve oxygen. Examples of these are:
- Reaction of heated sodium with chlorine
- Reaction of hydrogen with chlorine
- Reaction of sulfur with chlorine
A closer look at the processes that take place when sodium is burned into chlorine reveals that the metal atom gives up its outer electron and becomes a positively charged ion (cation). The released electron is taken up by the chlorine atom, which thus becomes a negatively charged ion (anion).
The reason for this lies in the so-called Octet Rule. This states that many atoms form molecules or ions in which the number of electrons on the outer shell corresponds to that of a noble gas. The distinguishing feature of the octet rule is that the outermost shell is completely occupied by eight outer electrons. This stable configuration is called the noble gas configuration.
Substances that can withdraw electrons from other substances (can oxidize them) are referred to as oxidizing agents. Thus, it is not only substances that can easily give off oxygen, but all substances that are able to accept electrons. On the other hand, all substances from which electrons can be easily withdrawn are called reducing agents.
In technology, the reduction of metal oxides with noble metals is of great importance. An example of such a reaction is the blast furnace process for steel production. Here, a redox reaction occurs between the iron oxide ores and the added coal as reducing agent. In the displacement reactions, it is noticeable that one can easily define the strength of oxidizing and reducing agents in a redox series. This is achieved by comparing their redox potentials. From this redox series, one can read the oxidizing or reducing character of a species.
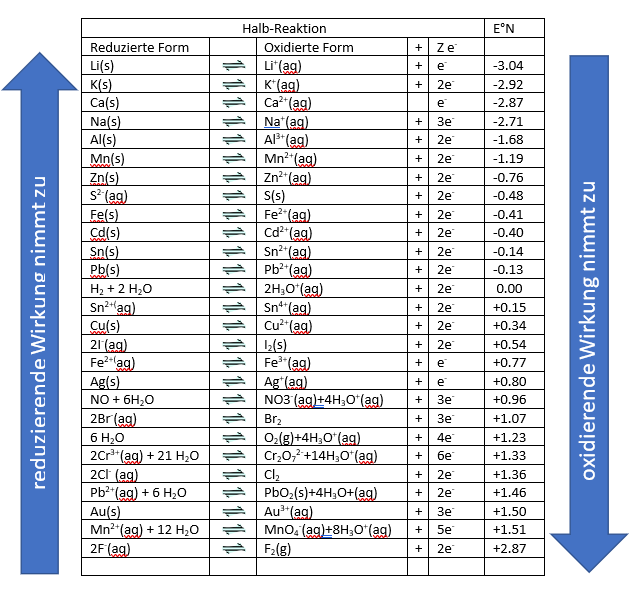
We can take the following meanings from this table:
- on the left side is the reduced form
- on the right side the oxidized form and the number of electrons emitted
- from top to bottom the oxidation potential increases
Each metal displaces the metal above it in the row from its salt solution. One often speaks of noble metals and non-noble metals.
The noble metals, which are chemically difficult to attack, are at the bottom of the voltage series: Silver (Ag), gold (Au), platinum (Pt). Accordingly, the base metals are located at the top: Sodium (Na), potassium (K), magnesium (Mg).
The reason for the occurrence of this redox voltage is therefore to be seen in the different behavior of the metals. Whether a metal acts as an oxidizing or reducing agent depends very much on the reaction partner.
In order to be able to measure a redox voltage accurately, a measuring cell is used. The construction of a measuring cell from a metal and its corresponding salt solution is called a half element. To measure voltage, two half-elements are connected together to form one element. The voltage occurring between the metal rods is used as a measure of the tendency of a substance to go into solution.
A cell composed of two half-elements is also called a galvanic cell. Chemical energy (in the form of the salt solutions) is converted into electrical energy (DC voltage).
The following components must be provided for the process-engineering integration of a redox measurement.
On the one hand, fittings are necessary to optimally integrate sensors into the process and to protect them. Therefore, shut-off valves and a suitable pipe routing must be provided in the delivery line. In lines with higher pressure, the sensors must be protected against pressure surges (pump run or valve shutdowns).
On the other hand, inert noble metal electrodes (usually made of platinum or gold) are used. These consist of a glass or plastic shaft, at the lower end of which a metal piece of certain shape is fused (Pt tip, Pt or Au pin). In conjunction with a reference electrode, a complete measurement chain is formed. The reference electrode (usually calomel -Hg2CI2 or silver – Ag/AgCI) has the task of providing a constant potential for potentiometric measurements, against which the potential of the metal electrode is measured. It consists of a glass or plastic shaft filled with the reference electrolyte and equipped with a dissipation system. A diaphragm in the shaft wall, provides the conductive connection between the electrolyte and the measured medium. A redox combination electrode contains metal and reference electrode in one shaft and thus represents a complete measuring chain. Only one installation point is required. When selecting the electrode, the respective area of application must be taken into account. The geometry as well as the materials used for the electrodes differ greatly depending on the area of application and are optimized for the respective application.
To obtain perfect transmission of the measurement signal, only special coaxial cables are used in pH and redox measurement technology. They establish the electrical connection between the sensor and the transmitter.
A transducer is also used. This has the task of processing the signal from the redox electrode. In the simplest case, this can be realized with a two-wire transmitter. Here, the electrode signal is converted into a signal of 4 – 20 mA and can thus be passed directly to a downstream PLC. Here the redox value is displayed and controlled.
In industry, redox measurements are considered in the following applications:
- Cyanide detoxification
- Chromate reduction
- Nitrite oxidation
- Swimming pool water monitoring
- Drinking water monitoring
- Indirect monitoring of oxidative biocides in e.g. cooling circuits
A large number of measuring instruments are available on the market for practical use. These can basically be divided into the two main groups of:
- Hand-held measuring instruments: enable rapid on-site determination and laboratory monitoring of test setups
as well as
- On-site transmitters or process control devices: combine the measurement of several parameters incl. process-specific processing of the measured values and their transmission via analog or digital data transmission.
subdivide.
The redox potential is of particular importance, for example, in the monitoring of swimming pool water. Here, the measured value serves as a physical variable for assessing the oxidizing or disinfecting effect of the disinfectant present (e.g. chlorine), taking into account the impurities present in the water at the time of measurement. The germicidal effect of oxidizing agents such as chlorine is based on the increase of the redox potential through their addition to the water to be treated. This actively causes a disturbance of the germ metabolism and the microorganism dies. It should be noted that the redox potential is directly dependent on the pH value. For this reason, both values are always recorded together. The higher the redox potential, the faster this process takes place. The so-called germination speed for 99.9% E-coli, for example, is a few seconds at a pH value of 7 and a redox potential of 750 mV.
Another application for the use of redox measurement is the indirect concentration monitoring of oxidative biocides in cooling circuits. In this application, non-oxidizing or oxidizing biocides are used, depending on the mode of operation and plant concept. A disadvantage of non-oxidizing biocides is that bacteria existing in the cooling water circuit can develop resistance to the biocide used. For the above reason, the alternating use of at least two different non-oxidizing biocides is recommended. The use of non-oxidizing biocides is usually based on the very good material compatibility of these products.
Oxidizing biocides, on the other hand, attack the materials used, but have the advantage that they can be monitored indirectly by measuring the redox potential. The measured redox value provides information about the disinfection effect currently prevailing in the circuit. When oxidizing biocides are used, a so-called corrosion inhibitor is added to the circulating water to prevent the corrosion damage that could potentially result.